Labeling Medicinal aerosols really should include at least the subsequent warning info on the label as in accordance with appropriate rules.GMP How do you keep up with the most recent traits and improvements in drug item specification advancement?Suspension is usually a colloidal method getting strong particles undissolved during the liquid phase.
different types of hplc systems Secrets
At IDEX Wellness & Science, we provide a broad selection of minimal tension fittings for systems that operate beneath one,000 psi, and also significant pressure fittings that provide future technology technological know-how to give you optimum general performance in even one of the most demanding applications.Then exploring related posts with speci
Indicators on hplc column dimensions You Should Know
Soon after reverse flushing, hook up the column inside the forward path and condition Using the standard mobile section in advance of using.The content of our website is often readily available in English and partly in other languages. Decide on your chosen language and We'll explain to you the written content in that language, if out there.An inef
How sterility testing for pharmaceuticals can Save You Time, Stress, and Money.
This minireview supplies an outline of this sophisticated area of latest very good production tactics (cGMP) determined by biopharmaceutical marketplace standards and summarizes the compendial and option swift microbial test solutions readily available for merchandise sterility and MycoplasmaSterility testing should be executed on remaining contain
A Review Of disintegration test apparatus working
b) if a residue remains, it consists only of a smooth mass having no good core which can't be pressed with a glass rod.Disintegration testing is a vital in-approach sign in oral reliable dosage (OSD) formulations mainly because it makes sure that the tablet or capsule will break down and release the Energetic pharmaceutical ingredient (API) in a we
 Barret Oliver Then & Now!
Barret Oliver Then & Now!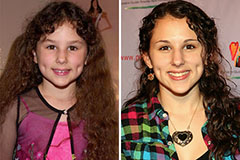 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now!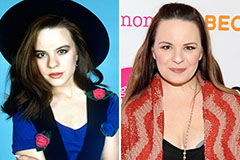 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Jurnee Smollett Then & Now!
Jurnee Smollett Then & Now! Marques Houston Then & Now!
Marques Houston Then & Now!